What is EPR?
Electron Paramagnetic Resonance (EPR), also known as Electron Spin Resonance (ESR), is a physical phenomenon of absorption of electromagnetic radiation by a special type of molecules when those are placed in a magnetic field. Such molecules, referred to as paramagnetic species, possess a non-zero total electron spin and can be likened to small magnets.
Paramagnetic molecules: free radicals and transition metals
A most common example of paramagnetic molecules is free radicals, the products of redox reactions. The radicals are typically chemically active and short-lived. Additionally, transition metals, often found in enzymes’ active sites, in the oxidation states where the total electron spin is not equal to zero, form another large group of paramagnetic species.
Paramagnetic species behaviour in an external magnetic field
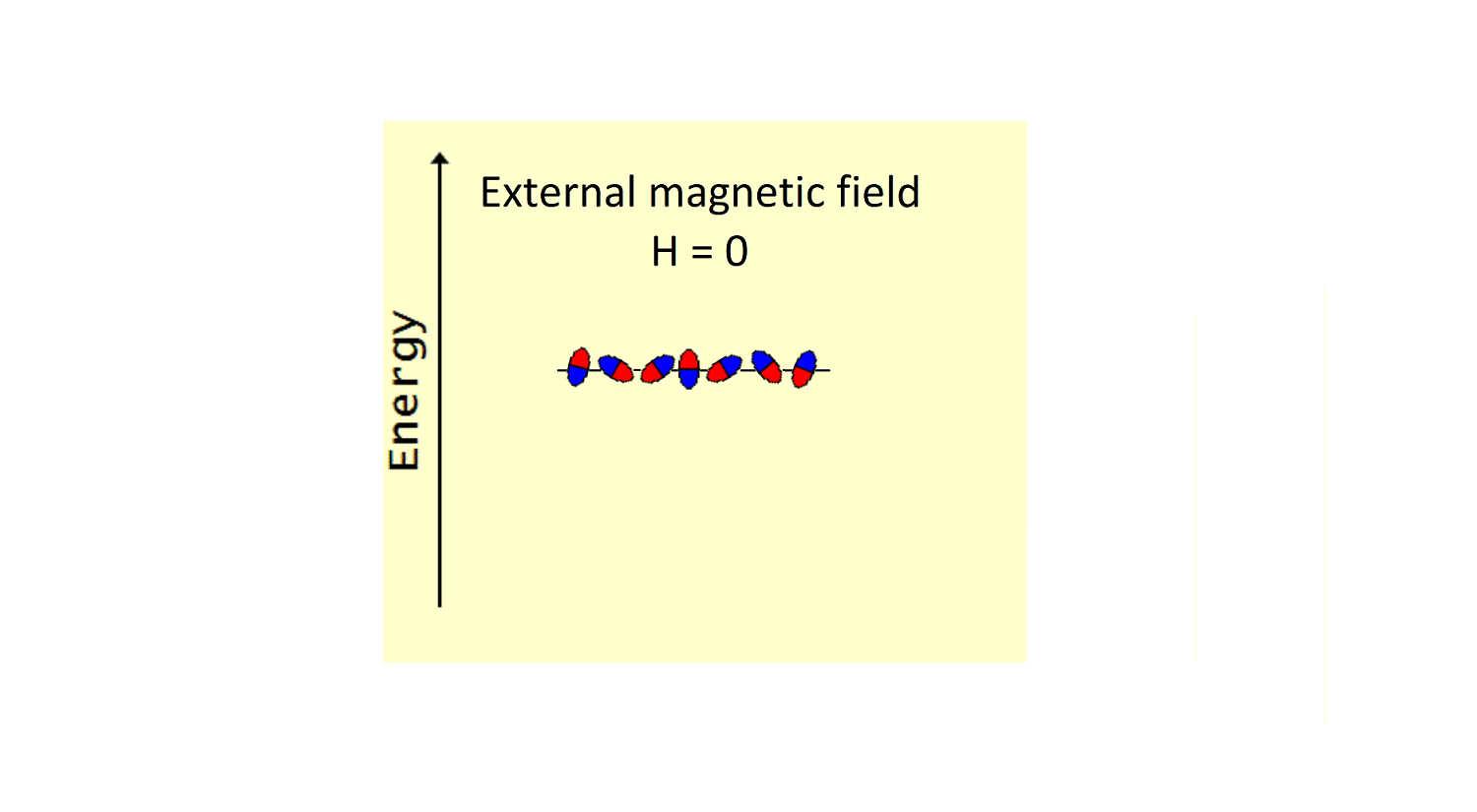
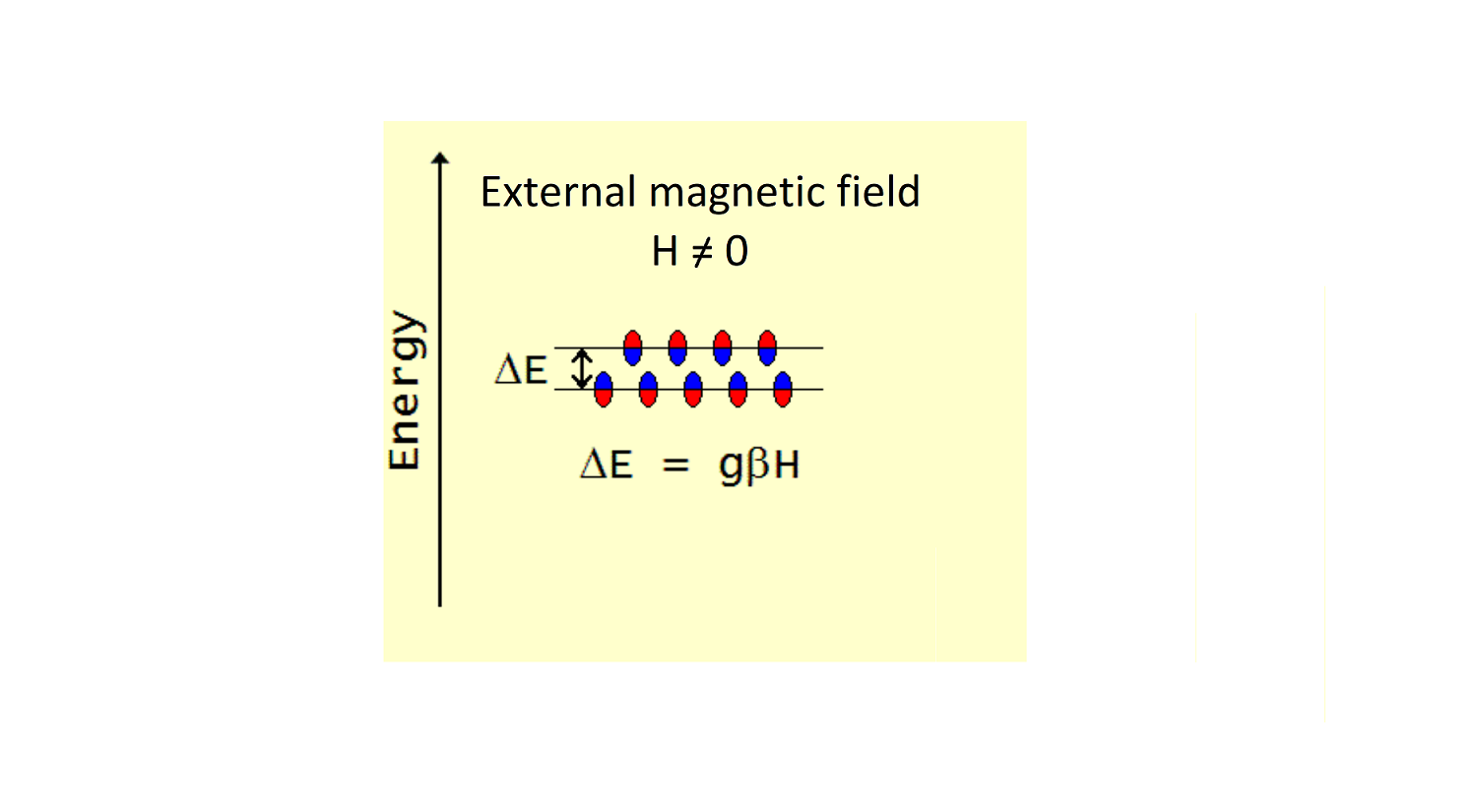
In a sample containing paramagnetic species, all non-zero spins (the tiny magnets) are oriented chaotically and have the same energy, as shown in Figure 1. Once an external magnetic field H is switched on, all the paramagnetic species, oriented chaotically before, are now re-oriented in two possible ways (Figure 2). Most of the magnets will align their magnetic axes along the direction of the external magnetic field. There will be also a fraction of magnets (smaller in number) aligned against that direction. The latter population, being less favourable, would have a higher energy. Therefore, these two groups of the paramagnetic species will have different energy, and it is said that the energy of paramagnetic species splits in the external magnetic field, the greater the field, the greater the distance between the two energy levels..
There are two coefficients of proportionality in this linear dependence of the energy split on the applied magnetic field (as shown in Figure 2). One is called the Bohr magneton β, which is a combination of fundamental constants and therefore is the same for all paramagnetic species. The other parameter is known as the g-value. It is specific to each paramagnetic species and can vary significantly depending on the nature of the species. For example, the g-value of free radicals is close to the value of 2, whereas the g-value of paramagnetic metals, for example of iron in haemoglobin, can be as high as 6.
Thus, the energy split in the figure above, when an external magnetic field H is applied, is ΔE = gβH.
The animation below shows what happens when we start increasing the external magnetic field while continuously irradiating the sample with an electromagnetic radiation. The frequency of the radiation 𝜈 is kept constant (in the microware radiation range), which makes the energy of the radiation quanta hν constant, too.
hν= gβHo
The spin transition between the levels requires energy which is taken from the microwave radiation. The microwave energy absorption can be measured quantitatively, this is exactly what an EPR spectrometer does.
The method of EPR spectroscopy has numerous applications in chemistry, biology, medicine.