Biomedical EPR Facility
Being part of the PSMD group of the School of Life Sciences, the Facility contributes to diverse research themes explored by the Group members
Electron Paramagnetic Resonance (EPR) spectroscopy is a method for detection, characterization and quantitation of paramagnetic species – the species with non-zero electron spins. Paramagnetic states of molecules are constantly formed during biochemical reactions in which electrons are transferred between molecules. EPR spectroscopy allows information about the nature and kinetics of formation and disappearance of such species thus providing invaluable insights into the nature and behaviour of these fascinating states, enabling a deeper understanding of their roles and functions in biological systems. There is a lot of websites describing the principles of EPR, but for a very basic understanding of the EPR phenomenon, you can visit our What is EPR page.
Our Biomedical EPR Facility
Our Facility houses a state-of-the-art X-band continuous wave (CW) machine - a Bruker E500 EPR spectrometer (Figure 1) - ideally suited for detecting paramagnetic states of biologically significant molecules. This includes proteins, enzymes, and other molecules, whether small or large, isolated or in complex environments such as cells or tissues.

Figure 1: Bruker E500 X-band EPR spectrometer
Key methodologies and our expertise
We offer EPR measurements at both low and room temperatures.
The low temperature EPR measurements is our main method of interrogating biological samples. For that, we currently use an Oxford Instrument helium system (Figure 2). Paramagnetic species are often transiently formed in biochemical reactions, often formed and gone over short time periods. To detect those, we freeze the reaction mixtures at specific times after the reaction is initiated. If such reactions are fast, the freezing time of the reaction mixture must be short.
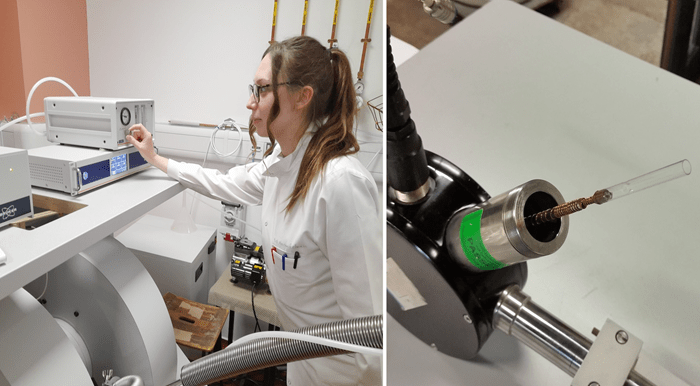
Figure 2: Left - Marina Rozman, PhD, is controlling the stability of the temperature set by the Oxford liquid helium system. Right – the heart of the cryostat – this is where the cold helium hits and cools the sample tube.
For the very short reaction times, we have a unique Rapid Freeze Quench (RFQ) apparatus (Figure 3) that allows freezing the reaction mixtures on a liquid nitrogen cooled metal surface. This makes our bespoke apparatus different from the most commonly used RFQ machines that employ isopentane as the cryogen (thus entailing a number of methodological challenges and outcome uncertainties). The University of Essex EIRA (Enabling Innovation: Research To Application) has produced a video of how our RFQ apparatuses might be used.
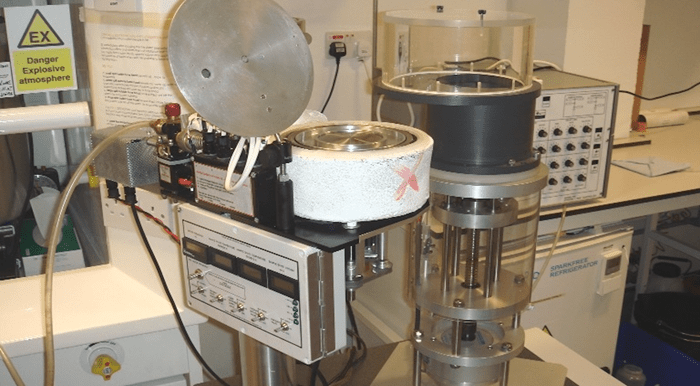
Figure 3: Our liquid nitrogen system to freeze quench samples together with the Update Instruments ram system to make solution mixtures.
Our RFQ apparatus can be operated in gas controlled conditions, for example – in an anaerobic environment (Figure 4).

Figure 4: Jacob Pullin, PhD, operates the RFQ system in oxygen-free argon atmosphere.
We also use our EPR spectrometer at room temperature, when the samples are in the liquid phase. The useful gadget for that is the Bruker AquaX system. With our bespoke attachment to an AquaX (Figure 5, right top), we can deliver a mixture of two reaction components to the spectrometer resonator and monitor the EPR spectrum change over time, from as early as ~1 s after mixing. Figure 5, left, shows the room temperature EPR spectra of the mixtures of human haemoglobin and hydrogen peroxide as observed in four similar experiments, marked by different colours of the spectra [1]. A tyrosine free radical is formed and quickly disappears in the reaction – the four sets of spectra in the figure show only the initial time points when the free radical EPR spectra intensities are relatively high. But those intensities can be accurately quantified over the whole time course of the reaction – and that allows measurements of the Tyr free radical kinetics, as shown in Figure 5, right bottom.
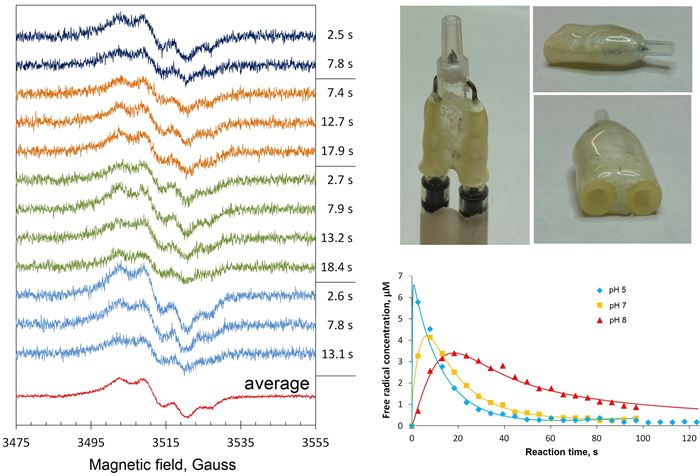
Figure 5: A bespoke attachment to the Bruker AquaX system, for measuring liquid samples, allows quantification of spectral change with a few seconds resolution. This allows accurate kinetic dependences measurements.
The liquid phase measurements at room temperature is particularly useful for the studies of protein spin labels and spin traps of protein-bound radicals and paramagnetic reactive oxygen species.
Spectra processing and simulation
We have exceptional expertise in decomposition of complex EPR spectra into several EPR signals caused by individual types of paramagnetic centres. In absolute majority of biochemical reactions which might be subjects to EPR spectroscopy investigations, more than one paramagnetic centres will be detected, and the overall EPR spectrum will be a superposition of different EPR signals. We use our spectra subtraction with variable coefficient procedure to obtain pure EPR line shapes of individual paramagnetic species and to measure their intensities in a set of samples, for example – in a kinetic series. The example given in Figure 6 shows how the intensity of the g=1.99 signal can be measured in spectrum B in units of this signal in spectrum A: it is clear that the signal’s intensity in B is 0.42 × (signal’s intensity in A).
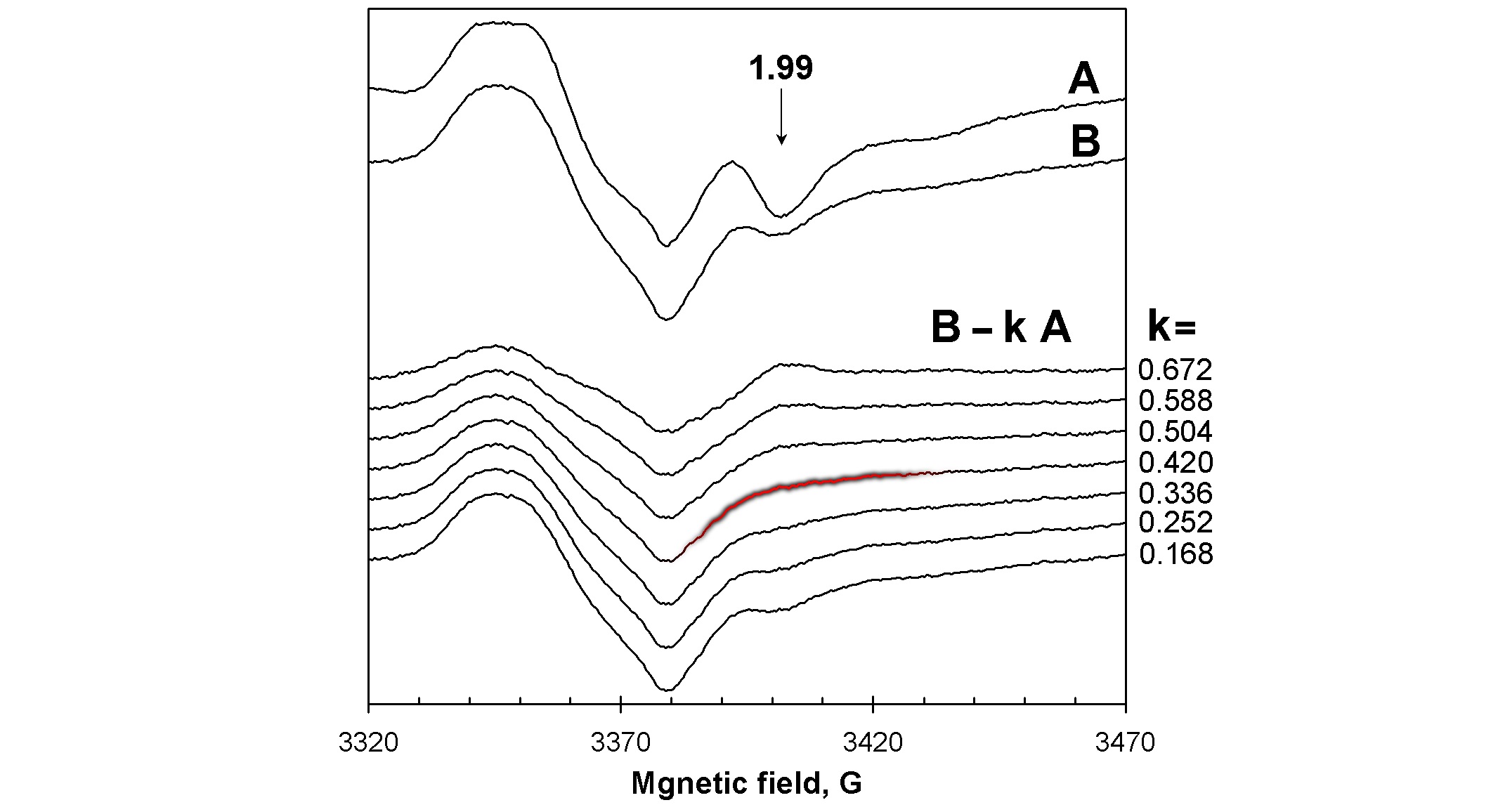
Figure 6: The spectra subtraction with variable coefficient procedure makes possible accurate measurements of EPR signals intensities when they are admixed with other signals. Here, the intensity of the g=1.99 signal in spectrum B is 0.42 of the intensity of this signal in spectrum A [2].
An important benefit the procedure gives is that a set of pure line shapes EPR signals extracted from the EPR spectra recorded in a course of a biochemical reaction can be employed in finding kinetics of each paramagnetic species. Such kinetic data, in turn, represent a solid base for testing hypotheses of the mechanism of the reaction.
To assign a pure line shape EPR signal to a specific paramagnetic centre, a simulation of the EPR signal should be employed. Numerous studies have demonstrated that when a radical is formed on a protein, tyrosine or tryptophan residues are the most probable sites of the radical. We have created and use in our research the two algorithms for simulating Tyr and Trp radical EPR spectra, both called TRSSA – Tyrosyl Radical Spectra Simulation Algorithm, TRSSA-Y, and Tryptophanyl Radical Spectra Simulation Algorithm, TRSSA-W. These algorithms significantly simplify the procedures of Tyr and Trp radicals’ EPR spectra simulation. Please find the details in our TRSSA page.
Scope of research
Since its foundation in 1996, the Biomedical EPR Facility has contributed to many publications on a broad range of subjects. The research themes might be categorised as follows:
- oxidative stress in animals and plants;
- nitric oxide in a variety of pathological conditions;
- electron transfer processes in proteins and enzymes;
- protein bound free radicals and high valence haem states in peroxidative reactions;
- the search for new blood substitutes;
- iron metabolism is cells.
We are open for collaboration
We are constantly looking for new PhD students and postdoctoral fellows. We also welcome collaborations with researchers from various disciplines, such as biochemistry, pharmacology, and materials science. Our primary focus is on advancing our understanding of biological systems using EPR spectroscopy. If you are interested in learning more about our collaborative opportunities and the publications that have resulted from these partnerships, please feel free to contact Dima Svistunenko at svist@essex.ac.uk.
References
[1] D. A. Svistunenko, A. Manole, J Biomed Res 2020, 34, 281-291.
[2] D. A. Svistunenko, N. Davies, D. Brealey, M. Singer, C. E. Cooper, Biochim Biophys Acta 2006, 1757, 262-272.



